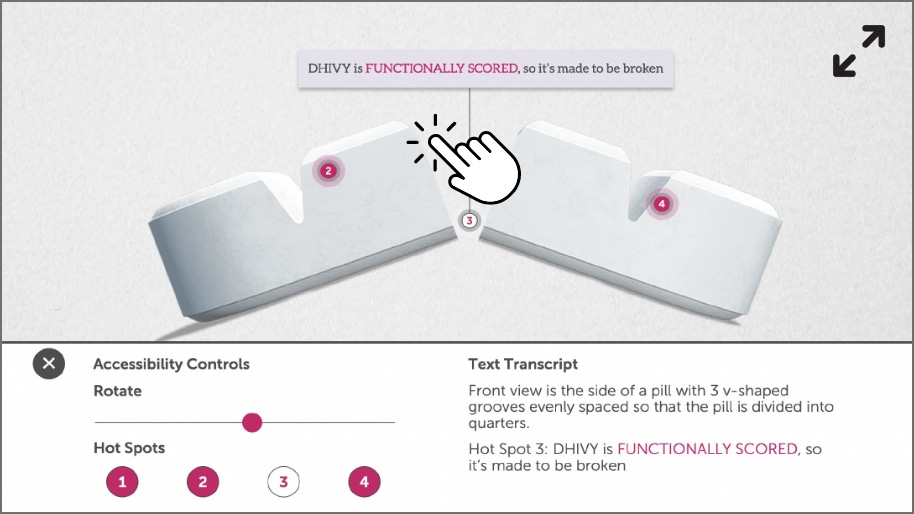
The DHIVY tablet was designed to break accurately1-3
DHIVY was developed by 2 neurologists to help meet the unique needs of patients with Parkinson’s disease
Click below to view the 3D Pill Model to understand more about DHIVY’s unique design.
DHIVY provides a range of segmented CD/LD amounts within a functionally scored tablet1,3

1 whole tablet
(25 mg/100 mg)

3/4 tablet
(18.75 mg/75 mg)

1/2 tablet
(12.5 mg/50 mg)

1/4 tablet
(6.25 mg/25 mg)
1/4 of DHIVY is the smallest segment available within a 25 mg/100 mg CD/LD tablet1,4
Read the open access publication on pill-splitting practices in Parkinson’s disease to understand the challenges patients and clinicians face.
Read PublicationDesigned with accuracy in mind1,2
Click here to see the unique tablet design in action.
Hear what Dr. Espay has to say about starting DHIVY.
Images do not represent the actual size of the tablet.
For patients switching to DHIVY from other 25 mg/100 mg immediate-release (IR) CD/LD tablets, start with a 1:1 conversion of the total daily dose.1
The recommended starting dosage of DHIVY is one 25 mg/100 mg tablet taken orally 3 times a day. Dosing with DHIVY should be individualized and adjusted according to clinical response and tolerability.1
A divided daily dose of CD/LD may yield clinical benefit for your patients5-7
The pharmacokinetic effects of smaller, more frequent doses of DHIVY were evaluated in a study7
(1/2 tablet every 2 hours vs 1 tablet every 4 hours)
Plasma levodopa concentration range declined by
51%
(P<0.0001)
Peak levodopa exposure declined by
44%
(P<0.0001)
Study Design: A 2-arm study was conducted under fasting conditions with 22 healthy adult volunteers. One IR CD/LD 25 mg/100 mg tablet was administered at 0 and 4 hours. After a washout period of 40 hours, half an IR CD/LD 25 mg/100 mg tablet was administered at 0, 2, 4, and 6 hours.7
Use performance dosing to maximize the therapeutic benefits of CD/LD1,5,6
DHIVY gives you the flexibility to individualize each dosing regimen.1
Individualize daily dosing
The total daily dose of DHIVY can be divided to meet patient needs.1
Fine-tune your titration strategy
Use quarter tablets to titrate your patients up or down.1
Provide prompt onset of action
DHIVY is an immediate-release formulation of CD/LD.1
References: 1. DHIVY. Package insert. Avion Pharmaceuticals, LLC; 2022. 2. Beach D. Accu-Break’s innovative tablet technology – 2016 advancements. ONdrugDelivery Magazine. 2016;69:39-40. 3. Center for Drug Evaluation and Research, Food and Drug Administration. Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation. US Department of Health and Human Services; 2013. 4. SINEMET. Package insert. Merck Sharp & Dohme Corp.; 2020. 5. Worth PF. When the going gets tough: how to select patients with Parkinson’s disease for advanced therapies. Pract Neurol. 2013;13(3):140-152. doi:10.1136/practneurol-2012-000463. 6. Tanner CM. Exploring the clinical burden of OFF periods in Parkinson disease. Am J Manag Care. 2020;26(12 suppl):S255-S264. doi:10.37765/ajmc.2020.88517. 7. AL-Sabbagh A, Testino SA, Shah D, et al. Variability of plasma levodopa conveniently reduced by more frequent administration of smaller doses of carbidopa/levodopa using a novel functionally scored formulation. Presented at: American Academy of Neurology Annual Meeting; April 2-7, 2022; Seattle, Washington. Presentation P7.11-002.
DHIVY — the CD/LD tablet made to be broken
DHIVY was designed with your unique needs in mind
ClickTap each
hot spot and drag to rotate the tablet to learn more about DHIVY's unique design.